Abstract
Introduction: Primary immune thrombocytopenia (ITP) is an autoimmune disorder characterized by a low platelet count resulting from platelet destruction and impaired platelet production. In the last decades, thrombopoietin receptor agonists (TPO-RAs) demonstrated a high rate of response in ITP and are licensed for use in persistent or chronic patients who have had an insufficient response to first line treatment with corticosteroids or Intravenous Immunoglobulin (IVIG). Some evidence suggests that switching to the alternate TPO-RA can be an appealing treatment option in patients with ITP for efficacy or safety issues; however, much of the evidence is retrospective and only a few prospective studies investigating alternate use of TPO-RAs. Hetrombopag is the first small molecule, nonpeptide oral TPO-RA developed in China and its efficacy has been demonstrated in a multicenter phase III trial (NCT03222843). Here we conducted a post hoc analysis of this phase III trial to assess the response in patients who switched from eltrombopag to hetrombopag.
Methods:A multicenter phase III study included a randomized, double-blind, placebo-controlled, 10-week treatment period (hetrombopag or placebo), sequentially followed by an open-label 14-week treatment period, during which patients who received placebo switched to eltrombopag with an initial dosage of 25 mg once daily and can be adjusted to a maximum of 75 mg once daily. Patients who completed eltrombopag 14-week treatment could directly continue additional 24-week hetrombopag treatment initiated at 2.5, 3.75, 5, or 7.5 mg once daily at the discretion of the investigator. Patients were assessed for efficacy and safety at every scheduled visit. In this post-hoc analysis, we investigated the response switching from eltrombopag to hetrombopag in ITP patients.
Results: All 63 (74.1%) patients who completed the initial 24-week treatment (10-week placebo and 14-week eltrombopag) entered a prespecified additional 24-week hetrombopag treatment period. At enrollment, the median age was 43 years (range 18-70) and 47 (74.6%) patients were female, the duration of ITP was three years or longer in 40(63.5%) patients. The median baseline platelet count before switching to hetrombopag was 69.0×109/L (range 6.0-278.0×109/L ) and one third patients had not achieved a sustained response (platelet count≥50×109/L) after eltrombopag treatment. The median dose of the prior eltrombopag at the time of switching was 50 mg daily.
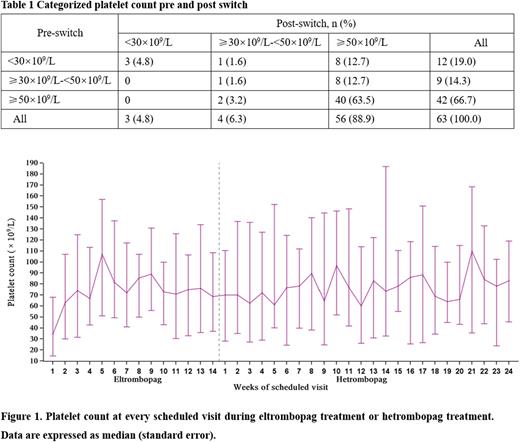
As of data cutoff on November 19, 2020, 58 (92%) patients completed the hetrombopag treatment period, the median exposure to hetrombopag was 169.0 days (range, 53.0-176.0); 28 (44.4%) patients received the maximum dose of 7.5 mg once daily and the final dose of the other 16 (25.4 %) patients was 5 mg once daily. Overall, 56/63 (88.9%) achieved a platelet response (platelet count≥50×109/L) after switching from eltrombopag to hetrombopag. Among 12 patients with prior platelet count lower than 30×109/L, eight patients (66.7%) achieved response. For nine patients with prior platelet count between 30×109/L and 50×109/L, eight patients (88.9%) achieved response (Table 1).
The median platelet count was 70 ×109/L after 2 weeks of switching and ranged between 62.5 ×109/L and 110 ×109/L across every scheduled visit during 24-week treatment with hetrombopag, highlighting that consistent platelet response could be maintained after switching from eltrombopag to hetrombopag. (Figure 1). The median maximum continuous duration of response was 78 days (range 8-165) and the median total duration of response was 104 days (range, 8-165) after switching to hetrombopag. In addition, 5(7.9%) patients received protocol-defined rescue therapy and 3(4.8%) patients had bleeding symptoms (All were grade 1 according to WHO bleeding scale) during hetrombopag treatment.
Conclusion:In this one of the largest evaluations of switching between different TPO-RAs. Hetrombopag was effective following prior eltrombopag treatment, with high response rates even in patients with inadequate response to the prior therapy. Our results confirm the reported efficacy of TPO-RA switch in ITP patients and the lack of cross-resistance between the two available TPO-RAs.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal